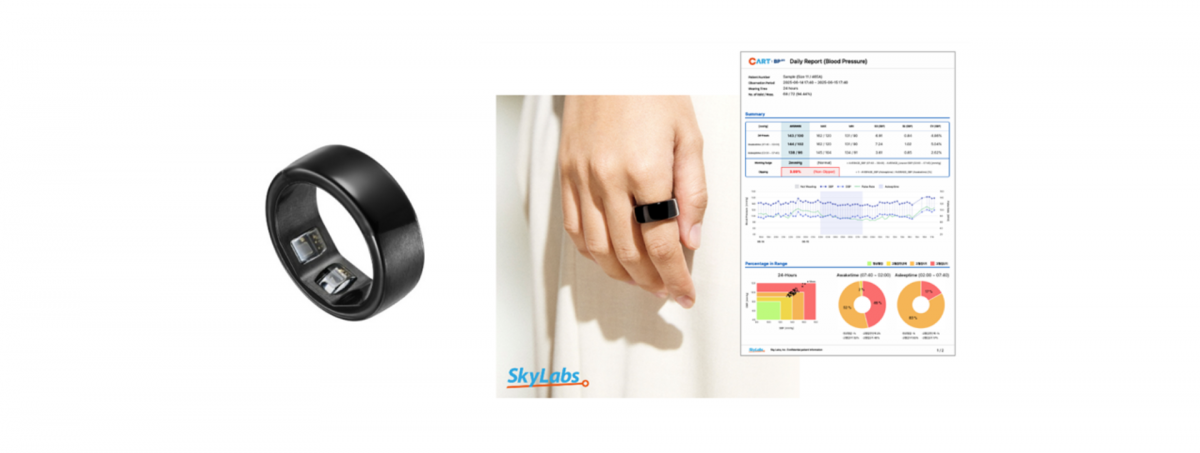
South Korea-based Sky Labs has entered an exclusive distribution agreement with Japan-based pharmaceutical company Otsuka Pharmaceutical to launch CART BP pro, a ring-type blood pressure monitor, in the Japanese hospital and clinic market, according to PR Newswire.
The agreement follows a memorandum of understanding signed between the two companies in December 2024. Sky Labs initially explored global expansion opportunities through Korea Otsuka Pharmaceutical, with the Korean affiliate facilitating formal discussions with Otsuka's headquarters.
Otsuka is a global pharmaceutical company providing medical solutions worldwide. By partnering with Otsuka and leveraging its expertise in the cardiovascular field and extensive distribution network, Sky Labs aims to reach a broader patient population and accelerate adoption of CART BP pro across Japanese hospitals and clinics.
The number of hypertension patients in Japan is approximately 43 million, of whom an estimated 29 per cent fail to achieve blood pressure control despite receiving treatment, and 33 per cent are believed to be unaware of their condition. Given these clinical requirements and the vast patient population, CART BP pro is expected to significantly contribute to the management of hypertensive patients in Japan.
CART BP pro has demonstrated technological prowess in the Korean market. Following medical device approval from the Ministry of Food and Drug Safety in 2023, it gained clinical recognition in 2024 through national health insurance reimbursement approval. The device is currently prescribed for 24-hour ambulatory blood pressure monitoring in over 1,700 medical institutions nationwide, surpassing 150,000 cumulative prescriptions within one year.
Jack Byunghwan Lee, chief executive of Sky Labs, said: "Starting with our entry into Japan, we will continue to validate our unparalleled blood pressure monitoring technology in the global market."
Learn more about the Sky Labs-Otsuka distribution agreement in the full story.














.png)

